Sysmex Life Science solutions to support lung cancer disease management
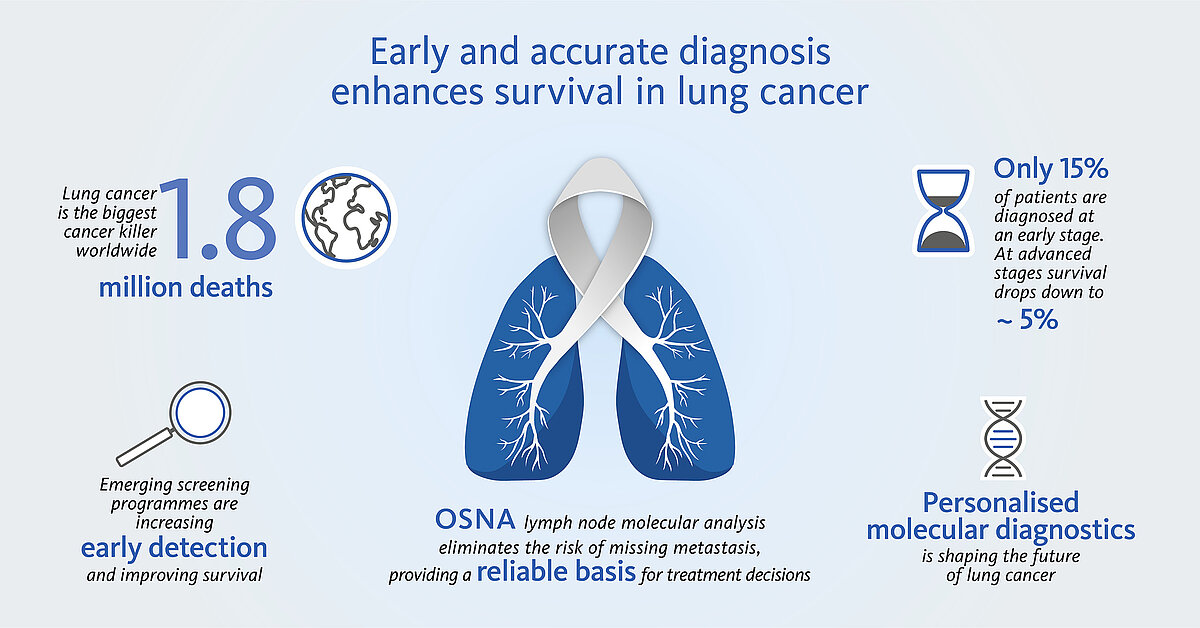
Lung cancer is still the leading cause of cancer-related death worldwide in both genders with more than 2 million new cases and 1.8 million deaths in 2020 [1]. The World Health Organization estimates that lung cancer deaths worldwide will continue to rise, largely as a result of an increase in global tobacco consumption (over one billion smokers in the world).
Today only 15% of all lung cancer cases are diagnosed at an early stage (5-year survival rate ~60%), but with screening programmes starting in many countries this number is expected to steadily increase [2]. The extent of nodal involvement is an important component in lung cancer staging and generally determines whether curative-intent surgery is feasible or not as well as supports further treatment decisions.
In the recent years, the advances have been dramatic in terms of development on precision medicine tools for clinical management especially of advanced stages of lung cancer. The genomic complexity and the large and still growing number of druggable oncogene drivers offer the opportunity to treat patients more personalised, thus improving survival outcomes.
Sysmex offers a set of molecular biology technology solutions supporting analysis of tissue and plasma to improve diagnostic accuracy.
Read the inspiring story of Liezl, a lung cancer survivor
She shares words of inspiration, hope and how this disease changed her life.
Diagnosis
In non-small cell lung cancer (NSCLC), surgery remains the best chance for a cure for early-stage patients. However, up to 30% of those patients experience recurrences after surgery. This is partly due to occult metastases in the lymph nodes that are missed by standard histology which analyses only a small portion of the node. This is a major concern since nodal status determines patient survival and guides intra- and post-operative treatment decisions.
Unlike histopathological methods, OSNA (one-step nucleic acid amplification) enables a fast, molecular whole node analysis. It provides nodal staging and prognosis accuracy as well as a reliable basis for personalised, fully informed treatment decisions.
Precision diagnostics for targeted therapy selection in lung cancer
Precision diagnostics are the key to selecting the right therapy at the right time for the right cancer patient.
Gene alterations play a crucial role during the onset and development of lung cancer. Therefore, reliable assessment of the tumour mutational status is decisive not only in selecting cancer patients eligible for targeted therapy, but also later in monitoring response to treatment and isease progression.
Nowadays, a range of medications targeting tumour-specific genetic alterations are available and can be prescribed instead of, or in combination with standard chemotherapies, immunotherapies and radiotherapies.
In non-small cell lung cancer (NSCLC), 2–7% of patients will harbour ALK rearrangements [3]. ALK gene is commonly rearranged with EML4 gene, producing the EML4-ALK fusion. ALK-driven tumours can be treated with crizotinib, a selective inhibitor of ALK and its oncogenic variants [4].
Abnormally elevated EGFR kinase activity can lead to proliferative diseases such as non-small-cell lung carcinoma (NSCLC), which accounts for 80–85% of all lung cancers [5].
There are several EGFR-inhibitor drugs in clinical use, for example, gefitinib and erlotinib in NSCLC. Approximately 10% of lung cancer patients show a rapid and dramatic response to these tyrosine kinase inhibitors (TKI) [6].
ROS1 rearrangements define a molecular subset of NSCLC and are seen in about 2% of patients [7]. NSCLC patients with ROS1 rearrangements have shown response to treatment with tyrosine kinase inhibitors, such as crizotinib [8].
Moreover, rearrangements involving the RET gene are recognised recurrent abnormalities seen in 1–2% of patients with lung adenocarcinomas [9].
The FGFR1 gene has shown to be amplified in approximately 9% of NSCLC patients [10] and has been associated with a poor prognosis [11].
To investigate such genetic changes, oncologists can make use of various molecular genetic tests which may support them in making personalised treatment decisions. Sysmex offers a range of products based on cutting-edge molecular biology technologies, supporting health care professionals and patients along the whole care pathway.
Molecular tests for therapy selection
CytoCell® FISH (Fluorescent In Situ Hybridisation) probes* targeting ALK, EML4, EGFR, ROS1, RET, FGFR1 genes are used to aid pathologists and clinicians to understand specific tumour information on key druggable genes and prognostic markers.
Download CytoCell pathology probes for lung cancer brochure
* CytoCell® FISH probes: for laboratory professional use only. Not intended for use as stand-alone diagnostics or companion diagnostics. Therapeutic action should not be initiated on the basis of the FISH result alone.
Manufacturer and Trademarks: CytoCell® FISH Probes (Cytocell Limited), Oxford Gene Technology IP Limited
PSS - Monitoring
More than 25% of early-stage lung cancer patients face disease progression after curative-intent surgery [12]. Thus, monitoring patients for signs of tumour recurrence, is of vital importance to secure the best possible long-term outcome for the patient.
In modern personalised oncology, certain somatic gene alterations have emerged as key biomarkers for disease progression, which are detectable in DNA from tumour tissue or circulating tumour-derived DNA (ctDNA) detected in liquid biopsy. At the stage of monitoring, tissue samples are often not available for analysis. However, liquid biopsies can be sampled repetitively with minimal patient distress and has shown to overcome limitations of tissue biopsies, such as missing tumour heterogeneity, inferior sample accessibility and long turnaround time.
Plasma-SeqSensei™ (PSS) RUO kits are next-generation sequencing-based (NGS) liquid biopsy assays intended for research of actionable mutations relevant in NSCLC, colorectal, breast and thyroid cancers as well as melanoma. Built on ultra-sensitive SafeSEQ technology, the PSS RUO kit portfolio aims to set a new standard for absolute determination of mutant molecules (MM) independent of real sample DNA input, thus offering researchers a cutting-edge tool to advance personalised oncology. Due to its ability to determine low variant allele frequencies, it is especially useful for minimal residual disease (MRD) detection and other applications requiring sequential measurement like adjuvant therapy response or recurrence monitoring.
* Plasma-SeqSensei™ LC RUO Kit is for Research Use Only. Not for use in diagnostic procedures.
Find out more about how Sysmex makes a difference in the battle to conquer lung cancer
Explore more
Resources
[1] Sung H, et al. (2021): Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. PMID: 33538338
[2] ESMO Essentials for Clinicians Thoracic Tumours (2019): Second edition
[3] Kwak, et al. (2010): N Engl J Med. 2010;363(18):1693-1703.
[4] Takaaki S, et al. (2010): Eur J Cancer. 2010; 46(10): 1773-1780.
[5] Jemal A, et al. (2006): CA Cancer J Clin 2006;56:106-30.
[6] Pao W, et al. (2004): Proc Natl Acad Sci USA 2004;101(36):13306-11
[7] Bergethon K, et al. (2012): J Clin Oncol 2012;30(8):863-70.
[8] Shaw AT, et al. (2014): 371(21), pp.1963-71.
[9] Kohno T, et al. (2012): Nat Med 2012;18(3):375-7.
[10] Macdonald D, et al. (2002): Acta Haematol; 107:101- 107.
[11] Seo AN, et al. (2014): Virchows Arch.;465(5):547-58
[12] Maurizi G, et al. (2015): Ann Thorac Surg.;100:918-924