Liquid biopsy – a flexible, convenient alternative to tissue sampling
Liquid biopsy uses bodily fluids to assess health status, most often employed for the detection of cancer presence in plasma. Tissue biopsy is the current gold standard, but it is not always available, or it can be very difficult to obtain (e.g. in lung cancer).
A liquid biopsy may be used to help find cancer at an early stage, as well as to help plan treatment, to find out how well treatment is working or if cancer has come back. Taking multiple samples of blood over time may also help doctors understand what kind of molecular changes are taking place in a tumour and how this might influence therapy.
What is liquid biopsy?
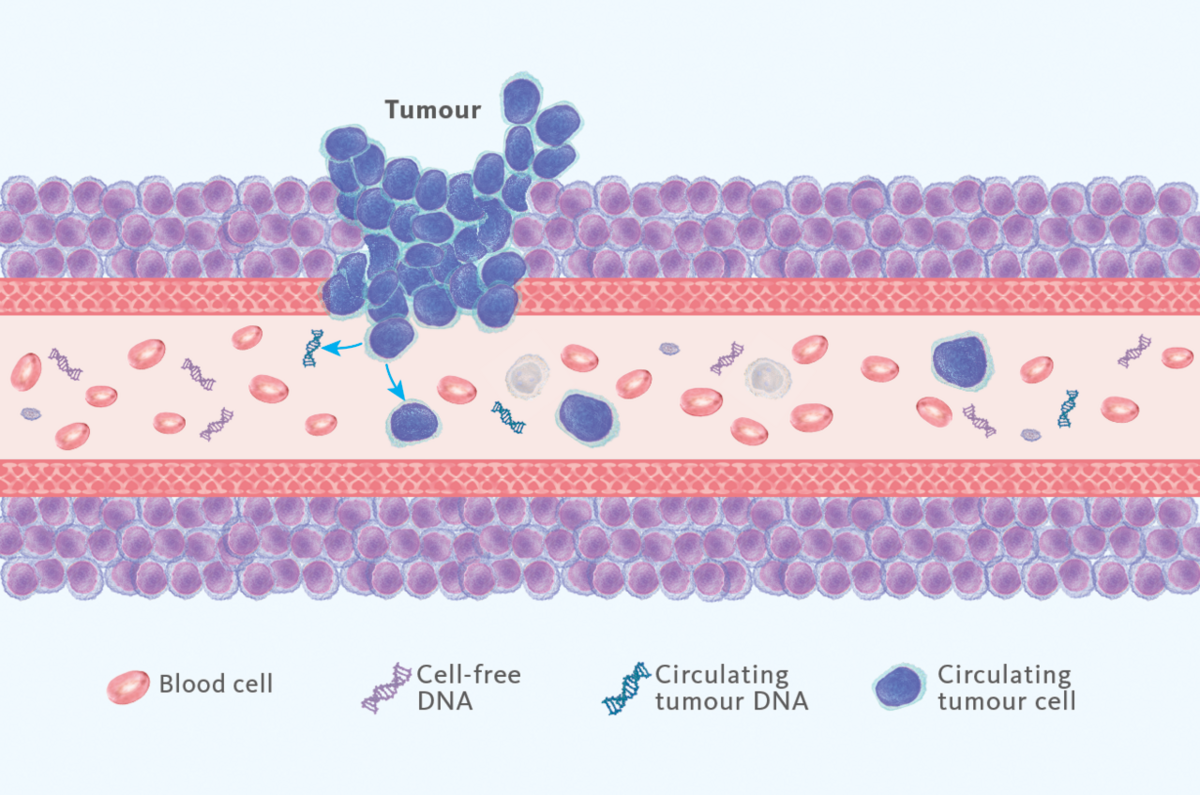
Liquid biopsy uses bodily fluids to assess health status, most often employed for the detection of cancer presence in plasma. As a target, circulating tumour DNA (ctDNA), circulating tumour cells and many other substances can be used (Fig.1), of which ctDNA is most broadly used one.
Tissue biopsy is the current gold standard, but it is not always available, or it can be very difficult to obtain (e.g. in lung cancer). It is also not practical to routinely use tissue samples for follow-up of patients. In contrast, liquid biopsy is a convenient and non-invasive method to obtain such information for advancing clinical studies. Because of this, liquid biopsy has now been included in some cancer guidelines already, including those for lung, colorectal and breast cancers.
Our OncoBEAM™ technology has been the most sensitive technology available by almost factor 20x versus other routine technologies such as PCR (polymerase chain reaction) and conventional NGS (next-generation sequencing). SafeSEQ, the technology building the basis of the new Plasma-SeqSensei™ product line, offers similar sensitivity combined with higher workflow convenience. The sensitivity is achieved by barcoding each sample, thereby reducing error rates by >100x.
Why use liquid biopsy?
- High concordance with tissue samples in most cancers
- Minimally invasive, tissue samples may be difficult to obtain
- More cost-effective than tissue biopsy
- More convenient for clinicians and patients
- Holistic tumour information (tissue samples may not be representative due to a sampling error)
- Faster turnaround time (TAT) compared to tissue sample handling
- Repeat sampling possible in contrast to tissue samples
- Real-time information
Why use Plasma-SeqSensei™?
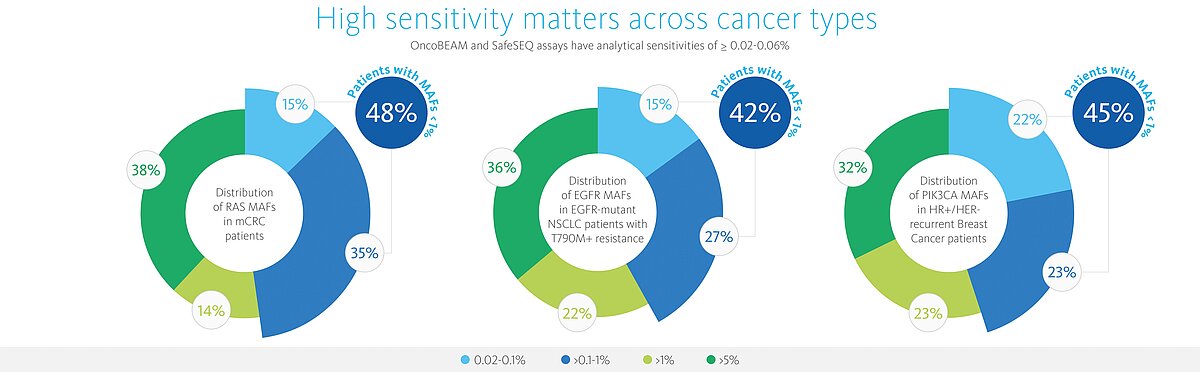
- Highly sensitive – able to detect 7 MM (mutant molecules) with 95 % confidence across all mutations (absolute quantification) and corresponding to a sensitivity of <0.07% MAF (mutant allele frequency, approx. 40 % of samples have MAF below detection limit of most technologies)
- High flexibility of between 2 - 16 samples/run
- High laboratory efficiency as several different types of samples may be processed in one run
- Standardised workflow, high reproducibility
- Fast TAT (2 days)
- Convenient software
Sysmex Inostics is the worldwide pioneer in Liquid Biopsy, established in 2008 and joining Sysmex in 2013. With more than 10 years’ experience in liquid biopsy, Sysmex Inostics is a trusted partner to leading pharmaceutical companies, advancing their efforts to bring the most effective personalised cancer therapies to global markets, from discovery through companion diagnostics.
Our liquid biopsy solutions
References
[1] National Cancer Institute (2023): liquid biopsy
[2] Schmiegel et al. (2017): . Mol Oncol. 11(2) : 208 – 219
[3] Saunders et al. (2016): . Annals of Onc 27 (6) : 149 – 206
[4] Vidal et al. (2017): . J Clin Oncol 35 (suppl 4S; Abstract 607)
[5] Oxnard et al. (2016): . J Clin Oncol 34(28):3375-3382
[6] Baselga et al. (2015): . Oral pres. SABCS, Abstract S 6 – 01